Dissecting the DAEN TGA Database of Adverse Events in Children 5-11
A leading research scientist has slammed “lying” news.com.au for spreading false reports of there being no serious adverse reactions to the Covid-19 vaccine among young children since the rollout.
The TGA is blatantly ignoring serious safety signals of this provisionally approved mRNA injection in the 5 to 11 year age group. Read to the end to discover that in Australia, in the first 5 weeks of the rollout, more than 80* of our healthy children have been administered either the incorrect dose of the vaccine or even the wrong mRNA / Covid-19 vaccine.
*Updated 28 Feb 2022
Methodology
In order to extract this data, the author used the DAEN database, searching the date range for the four medicines selected using the search criteria listed above and manually adding up each individual case in the listed report “product administered patient of inappropriate age”.
Introduction - Phase 1 Dose-Finding Clinical Trials
Phase I Clinical Trials were conducted on a small number of children to determine the dose that would be selected for the 5 to 11 year olds to follow onto Phase II/III Clinical Trials. Dose-finding Phase I clinical trials conducted under controlled conditions halted the 30 μg dose in the 5 to 11 year old cohort due to toxicity and safety issues in less than 16 trial participants in this age group.
Published online 2021 Nov 9, the paper Evaluation of the BNT162b2 Covid-19 Vaccine in Children 5 to 11 Years of Age states:
Adverse events from the first dose through 1 month after the second dose were reported by 50.0% of those who received two 30 μg doses in the 5 to 11 year age group.
So why are we giving this dose to our kids? Why is it not a significant safety concern? And what does the actual post-marketing surveillance data show us on the effects of this mRNA injection on children in the ages 5 to 11?
Background…
On 3 February 2022, news.com.au, an Australian website owned by News Corp Australia, reported to its 9.6 million unique readers that a leading pediatrician has slammed “lying” social media users for spreading reports of serious adverse reactions to the coronavirus vaccine among young children online.
Australia’s medicines regulator says as of January 23 it has received 240 reports of adverse events in the jab rollout for five to 11-year-olds, the vast majority of which have been mild and expected.
This article’s intention is to dissect this data, compare it to similar overseas data, and unravel it in order to let the reader decide which side is actually perpetuating the lie.
“Because the sun comes up and five minutes later someone has a car crash, is that a causative relationship? My God, let’s introduce a little bit of intelligence and reasonableness into this discussion.” Prof Booy told news.com.au.
So where did these reports come from? The Database of Adverse Event Notifications (DAEN) - medicines allows you to search adverse event reports for medicines including vaccines received by the TGA. These reports came from a wide range of sources, including members of the public, GPs, other health professionals, and the therapeutic goods industry.
When to report AEFIs
Most reactions to vaccines are like low-grade fever and pain at the spot where the needle went in. They are usually mild and short-lived. These reactions do not require special treatment or reporting. You should report to the state and territory AEFI contacts below if you have concerns about a reaction that:
appears to be getting worse
does not fit the common reactions for that vaccine
How to report an AEFI
Vaccination providers can report an AEFI or a defect with a vaccine via the TGA website.
You should also report AEFIs to State and Territory contacts:
ACT: ACT Health 02 6205 2300
NSW: NSW Health 1300 066 055 (to connect to your local public health unit)
NT: NT Health 08 8922 8044
QLD: Queensland Health 07 3328 9888, or complete an AEFI initial report form on the Queensland Health website
SA: SA Health 1300 232 272 (Immunisation section)
TAS: Report direct to the TGA
VIC: SAEFVIC 1300 882 924 or the SAEFVIC website
Reporting an AEFI via the TGA Website
The reporting system has the capability to record the following as a minimum:
Whether the vaccine was suspected to play a role in the adverse event
Whether that adverse event is life-threatening, lead to hospitalisation, disability or death.
Duration of the event, reaction details including medical records, and concomitant medication details.
In fact, this seven-step process is lengthy, detailed, and quite thorough. It took almost 45 minutes just to walk through this process.
A spokeswoman for the Therapeutic Goods Administration (TGA) told news.com.au there had been a “number of individual reports of other types of reactions, none of which represents a signal for any serious reaction”, and that those reports – published on a two-week time delay on the regulator’s public Database of Adverse Event Notifications (DAEN) – do “not mean that the vaccine caused the reported event”.
Since we are able to search this data for ourselves let’s dive into those two statements:
none of which represents a signal for any serious reactions
do not mean that the vaccine caused the reported event
Looking at the DAEN Data
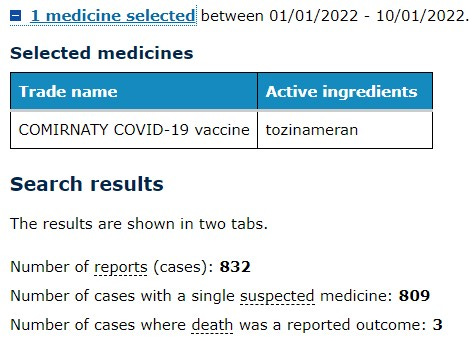
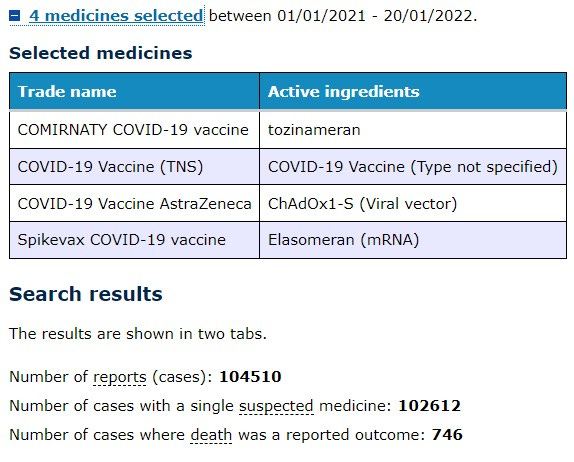
For the interest of consistency, the data from the DAEN database in this analysis is for the one vaccine name “COMIRNATY COVID-19 vaccine (active ingredients: tozinameran)” as listed in the database as it is the only vaccine provisionally approved for use in 5-11 year olds in Australia.
Pfizer’s Comirnaty vaccine was approved for use in adults on 25 January 2021, so at the time of writing there are almost 12 full months of data on this vaccine available to search in the DAEN database - noting that the database is two weeks behind real-time as mentioned by the TGA spokesperson. I have included the last 12 months of data in this analysis.
The following searches were therefore based on dates entered as 25 Jan 2021 - 20 Jan 2022.
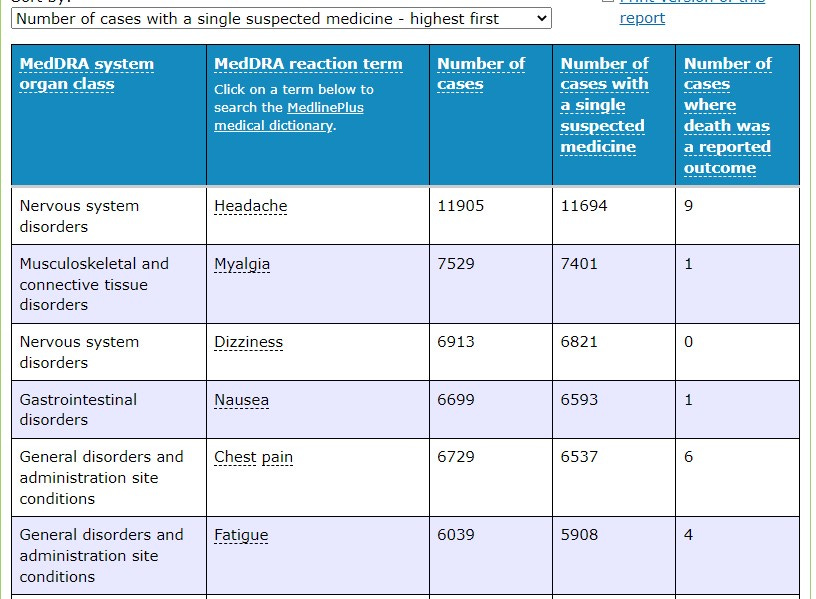
The first thing I did notice is that although often cited as the most common ‘mild’ reaction ‘headache’ appears to also have 9 cases where death was reported as an outcome.
The fifth most common general disorder is chest pain. Interestingly when you try to search further into the query for this year-long date range to determine ‘all cardiac events’ for this drug the system crashed. I tried 4 times to no avail.
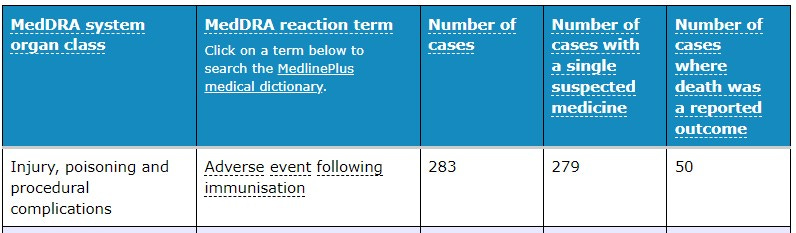
In that same time period, the reaction term associated with the highest number of cases where death was reported as an outcome was purely listed as an Adverse event following immunisation. It would be difficult to comprehend that the person filling out this report would have selected the drug Comirnaty as the suspected medicine, then go on to report it as an ‘Adverse event following immunisation’ ‘leading to death as a reported outcome’ had they not believed that “the vaccine caused the reported event”.
“Children tolerate vaccination better than teenagers and adults and we’re pleased with the real-world evidence of safety in over 10 million North American children and close to one million Australian children.”
Says Prof Booy, although the figures are somewhat misleading…
As of January 26, 2022, the CDC recorded: 8.3 million US children ages 5-11 have received at least one dose of COVID-19 vaccine and 5.7 million US children ages 5-11 are fully vaccinated. 16.4 million US children and adolescents ages 12- 17 have received at least one dose of COVID-19 vaccine and 13.7 million of US children and adolescents ages 12- 17 are fully vaccinated.
Source: https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-vaccination-trends/
Real-world data in VAERS is only grouped by ages 6-17 and does not differentiate between ‘children and teenagers’ therefore it is not possible to determine whether there are fewer or more cases of adverse event reports for children vs teenagers using the CDC data. A total of 37,786 reports were made in this combined age bracket representing 1 in every 653 children vaccinated with at least one dose are reported to the CDC as having a reaction that resulted in an adverse event being filed, 3,046 required hospitalisation, had a life-threatening injury, or resulted in permanent disability or death.
Source: https://wonder.cdc.gov/
The TGA said reports of more serious effects in US children were “extremely rare” with 100 reports from 8.7 million vaccine doses.
It is extremely unclear where this figure of 100 is actually pulled from and was there actually “10 million doses” or 8.3 million doses or “8.7 million doses”?
Perhaps they are referring to myocarditis and pericarditis, the two serious side effects recognised as being linked to the mRNA Covid-19 vaccines.
Nope. That’s not 100. Perhaps they only consider death as a ‘more serious outcome’ of which 74 reports were made to VAERs in the 6-17 age group.
“The most common were fever, vomiting and in some cases seizures,” it said.
Actually, the most commonly reported side effect alarmingly is ‘chest pain’ in 6-17 year olds following the mRNA Covid-19 vaccines representing 38.44% of all reports.
The TGA spokesperson also advises news.com.au that “none of which represents a signal for any serious reactions”
Myocarditis in Children
Myocarditis is consistently reported in the mainstream media as being mild and this news.com.au article goes on further to use the term ‘temporary’.
For example, there was a concern in older teenage boys that one in 10,000 developed a temporary myocarditis, inflammation of the heart after the second dose of an mRNA vaccine.
Further, the TGA’s own website publishes a COVID-19 vaccine weekly safety report where it states “Myocarditis is a known but very rare side effect of Comirnaty (Pfizer) and Spikevax (Moderna). It is usually temporary, with most people getting better within a few days.”
The definition of ‘very rare’ in relation to adverse events according to the WHO is as follows:
Source: https://www.who.int/medicines/areas/quality_safety/safety_efficacy/trainingcourses/definitions.pdf
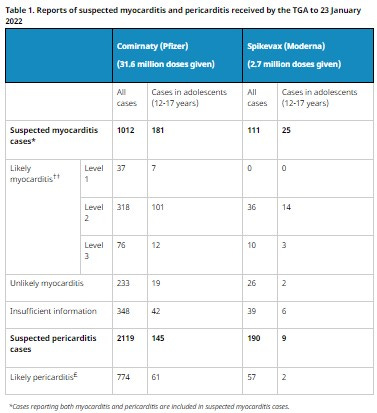
Again, the TGA’s weekly report states:
Myocarditis is more commonly reported after the second dose in teenage boys (11 cases per 100,000 Comirnaty doses and 16 cases per 100,000 Spikevax doses) and men under 30 (6 cases per 100,000 Comirnaty doses and 12 cases per 100,000 Spikevax doses).
Which converted into the same denominations used by the WHO are:
Comirnaty 1 / 9,009 and
Spikevax 1 / 6,250
Which would be ‘rare’ but certainly not ‘very rare’.
They conveniently add a table so we can see the breakdown:
Source: https://www.tga.gov.au/periodic/covid-19-vaccine-weekly-safety-report-27-01-2022#section-1433
If Myocarditis isn’t very rare, can we believe them when they say it is also “temporary”?
Not according to the countless individual stories documented here, a few stories in and one man is complaining that his chest pain has lasted 6 months:
This is just one case you say - yes that is true but there are 164 other similarly heartbreaking stories of long term disability and victim shaming documented here:
https://www.instagram.com/jab_injuries_australia/?hl=en
What do the experts think? Is Myocarditis temporary?
Though each case is unique, fortunately, the prognosis of the disease is mostly positive. In general, uncomplicated myocarditis often heals within several weeks. In other cases, the inflammation and damage of the heart may need longer to heal so this may take a few months or even a year to recover completely. In a minority of cases, the disease causes serious and irreversible damage to the myocardium, leading to a persistent heart failure even though if the inflammation has relieved – therefore, long-term medical therapy or even heart transplantation is suggested.
According to the MayoClinic:
Myocarditis in children
When children develop myocarditis, they might have signs and symptoms including:
Fever
Fainting
Breathing difficulties
Rapid breathing
Chest pain
Rapid or irregular heart rhythms (arrhythmias)
Complications
Usually, myocarditis goes away without permanent complications. However, severe myocarditis can permanently damage your heart muscle, possibly causing:
Heart failure. Untreated, myocarditis can damage your heart's muscle so that it can't pump blood effectively. In severe cases, myocarditis-related heart failure may require a ventricular assist device or a heart transplant.
Heart attack or stroke. If your heart's muscle is injured and can't pump blood, the blood that collects in your heart can form clots. If a clot blocks one of your heart's arteries, you can have a heart attack. If a blood clot in your heart travels to an artery leading to your brain, you can have a stroke.
Rapid or irregular heart rhythms (arrhythmias). Damage to your heart muscle can cause an arrhythmia.
Sudden cardiac death. Certain serious arrhythmias can cause your heart to stop beating (sudden cardiac arrest). It's deadly if not treated immediately.
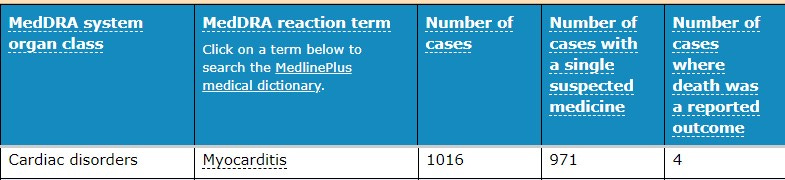
A recent PUBMED study by Dr. Peter McCullough used data from the Vaccine Adverse Events Reports System (VAERS) to examine cardiac adverse events (AEs)—specifically myocarditis—following the injection of the first or second dose of one of the three experimental COVID-19 “vaccines” currently being administered under emergency use authorization (EUA) in an “unprecedented fashion.” The study revealed it is safe to assume the reporting of heart-related complications, including myocarditis, in VAERS is not rare, but instead, just the “tip of the iceberg.”
Co-authored with Dr. Jessica Rose, the peer-reviewed, approved, and published paper, titled “A Report on Myocarditis Adverse Events in the U.S. Vaccine Adverse Event System (VAERS) in Association with COVID-19 Injectable Biological Products,” was abruptly removed from publication after being fully accepted with executed publishing agreements.
Within 8 weeks of the public offering of COVID-19 products to the 12-15-year-old age group, we found 19 times the expected number of myocarditis cases in the vaccination volunteers over background myocarditis rates for this age group.
In addition, a 5-fold increase in myocarditis rate was observed subsequent to dose 2 as opposed to dose 1 in 15-year-old males.
COVID-19 injectable products are novel and have a genetic, pathogenic mechanism of action causing uncontrolled expression of SARS-CoV-2 spike protein within human cells.
When you combine this fact with the temporal relationship of AE occurrence and reporting, biological plausibility of cause and effect, and the fact that these data are internally and externally consistent with emerging sources of clinical data, it supports a conclusion that the COVID-19 biological products are deterministic for the myocarditis cases observed after injection.
Someone should let Prof Booy know that the ‘causative and temporal relationship’ has been established.
Yet, news.com.au states “The TGA said reports of more serious effects in US children were “extremely rare” with 100 reports from 8.7 million vaccine doses.”
100/8700000
1/87,000
At least they got the definition correct this time - but what about those numbers?
We used the VAERS adverse event reporting system (the equivalent to Australia’s DAEN database) to confirm using these search parameters:
And we found 900% more cases of myocarditis than reported by the TGA spokesperson …
Are Fewer Than 1% of Vaccine Injuries Reported to VAERS? is a question for an entirely separate article. However, you start to get the picture of how loose the authorities are with the numbers and definitions without delving into under-reporting and the failure of VAER’s as a post-marketing safety surveillance tool.
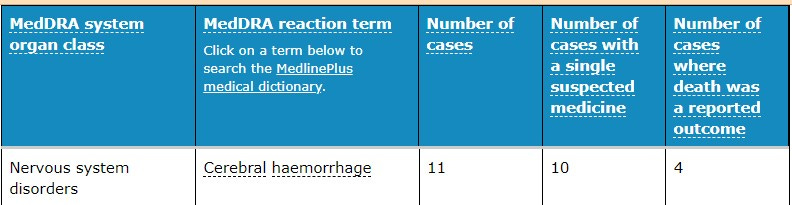
Moving on to other ‘rare’ and ‘temporary’ side effects of the genetic vaccines. Cerebral hemorrhage is uncontrolled bleeding in the brain. It can occur from an injury or as a result of a leaky or burst blood vessel. This can happen when a blood vessel gets weakened enough that its wall can no longer withstand the pressure of the blood flowing through it.
Genetic Covid-19 Vaccines in Children 5 - 11 Years
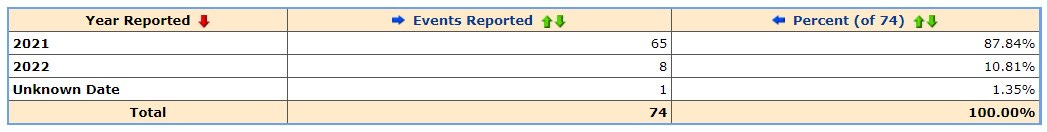
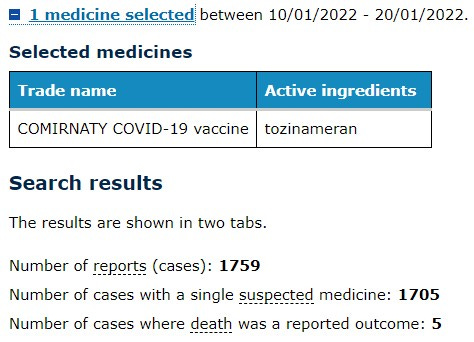
We know that the vaccines were rolled out to this age group on the 10 Jan so let’s look at the previous 10 days as a baseline to establish how many adverse events were being reported in a similar period.
Compared to the following 10 days after the vaccine rollout commenced in the children aged 5-11.
The increase could be attributed to the New Years Day holiday of course so we separately downloaded each report where age was provided*
(*There are numerous reports where age is not given and these have not been included in our analysis).
We extracted a total of 169 reports.
“To January 23, 2022, we have received 240 reports from approximately 657,000 Comirnaty doses administered in this age group,” it said.
“The most common reactions reported included fainting, chest pain, nausea, vomiting and headache. We have received four reports of suspected myocarditis and/or pericarditis in this age group.
We assume then that the remaining 71 reports are either in the group where no age is recorded in the publicly searchable part of the database and the remaining ones are yet to be entered.
In our analysis of the 169 reports that we could search the following were the most frequently reported side effects:
Chest Pain - 22 cases
Nausea - 22 cases
Headache - 16 cases
Dizziness - 15 cases
Pericarditis - 3 cases
Tachycardia - 3 cases
Myocarditis - 2 cases
Chest pain is the most commonly reported side effect of administering a 10ug dose of Comirnaty vaccine to 5-11 year olds.
Heart-related causes of chest pain include:
Heart attack. A heart attack results from blocked blood flow, often from a blood clot, to the heart muscle.
Angina. Angina is the term for chest pain caused by poor blood flow to the heart. This is often caused by the buildup of thick plaques on the inner walls of the arteries that carry blood to the heart. These plaques narrow the arteries and restrict the heart's blood supply, particularly during physical activity.
Aortic dissection. This life-threatening condition involves the main artery leading from the heart (aorta). If the inner layers of this blood vessel separate, blood is forced between the layers and can cause the aorta to rupture.
Inflammation of the sac around the heart (pericarditis). This condition usually causes sharp pain that gets worse when breathing in or lying down.
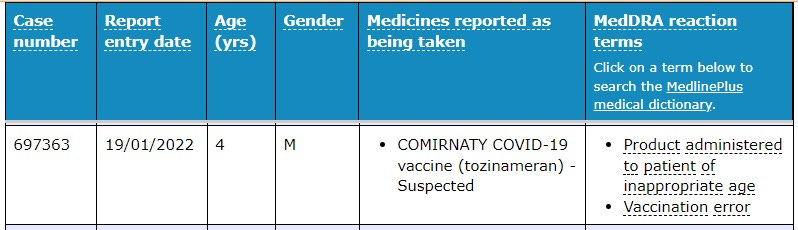
Product administered to a patient of inappropriate age
Also recorded as “Wrong product administered” and “Product administered to patient of inappropriate age” was a group of adverse event cases recorded in the age group 5 to 11.
There were a total of 83 children injected with the wrong product.
This is vitally important information so please follow along:
The vaccine dose approved by the TGA for children aged 5 to 11 is the same safe and effective vaccine used for other age cohorts, however is one-third the dose approved for those aged 12 and over.
The Pfizer vaccine for 5 to 11-year-olds will be distributed to vaccine providers in different packaging to the vaccine approved for people 12 and over, and will be clearly differentiated by being dispensed from orange-capped vials instead of grey or purple capped vials.
Source: https://www.health.gov.au/ministers/the-hon-greg-hunt-mp/media/tga-provisionally-approves-pfizer-covid-19-vaccine-for-5-to-11-year-olds
The hypothesis is that the ‘wrong product’ or ‘product administered to the inappropriate age’ means that the patient was injected with the adult/teenage dose. Once the disposable orange-capped vials are opened, they are virtually indistinguishable from the other vials as they are all multiple-use and all have grey stoppers.
What does an adult dose in a child look like?
A phase 1, dose-finding study and an ongoing phase 2-3 randomized trial are being conducted to investigate the safety, immunogenicity, and efficacy of two doses of the BNT162b2 vaccine administered 21 days apart in children 6 months to 11 years of age.
Evaluation of the BNT162b2 Covid-19 Vaccine in Children 5 to 11 Years of Age.
Results: During the phase 1 study, a total of 48 children 5 to 11 years of age received 10 μg, 20 μg, or 30 μg of the BNT162b2 vaccine (16 children at each dose level).
At each planned dose level (10 μg, 20 μg, and 30 μg), four sentinel participants received injections of BNT162b2, with vaccination followed by a planned 2-day pause. If no safety events of concern were noted, the remaining 12 participants in the group were vaccinated.
In laymen’s terms - four children were selected to receive the dosage prior to injecting the remaining 12 participants with that dosage.
What happened when they gave the adult / teenage 30 μg of the BNT162b2 vaccine dose (grey or purple capped vials) to four children in the 5 to 11 years of age group?
More side effects…
Fever was more common in the 30-μg dose-level group than in the 10-μg and 20-μg dose-level groups after the first and second doses (Fig. S1B).
All four sentinel participants in the 30-μg dose-level group who received the second 30-μg dose had mild-to-moderate fever within 7 days; the remaining 12 participants in the 30-μg dose-level group received a 10-μg second dose approximately 1 month after the first dose, as recommended by the internal review committee after selection of the phase 2–3 dose.
In laymen’s terms, they stopped administering the 30-μg dose after the reactions in the 16 participants demonstrated a safety signal that triggered an internal review committee to discontinue this dose during the Phase 1 trial.
So, when the TGA spokesperson told news.com.au there was “no signal for any new safety concerns arising from the limited number of adverse event reports received for children” she obviously hasn’t reviewed the data where MORE CHILDREN IN AUSTRALIA HAVE BEEN ADMINISTED THE INCORRECT DOSE THAN WERE SUBJECTED (AND HALTED) IN THE DOSE FINDING CLINICAL TRIAL.
If children subjected to an unsafe dosage as determined in Phase I controlled clinical trials isn’t a NEW SAFETY CONCERN then I do not know what is!
In the Phase I dose-finding study adverse events from the first dose through 1 month after the second dose were reported by 50.0% of those who received two 30 μg doses.
There is an obvious vaccination error recorded here … this patient is not even in the age group provisionally approved for.
The following may well be the first stroke in a 5-year-old following the Covid-19 vaccination.
There have been reports of a young woman who had a headache for a few days, before developing a fatal cerebral haemorrhage ten days after vaccination with the ChstraOx1 nCoV-19 vaccine from AstraZeneca.
Although, one would think this also represents an early safety signal worth investigating.
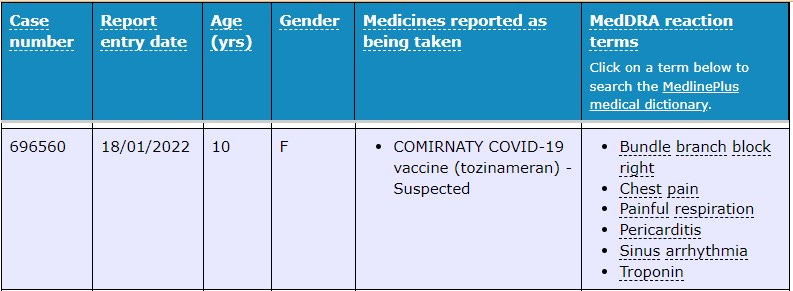
Right bundle branch block comes from a problem with the heart's ability to conduct electrical signals.
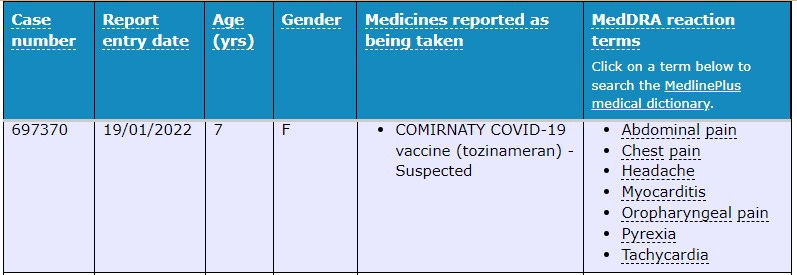
Again, our old fiend the ‘mild, temporary and very rare” myocarditis presented in a 7 year old female with other symptoms including tachycardia, chest pain and a headache.
Prof Booy said the TGA had 25 experts working on surveillance of side effects “to have the best quality and the most rapid analysis of any reported serious side effect”.
With 240 reports in children aged 5 to 11 in the first 10 days, each of these experts must be overwhelmed having received 9.6 reports each - or roughly one a day since the rollout began.
With evidence that the second dose of Comirnaty is associated with a higher number of adverse events and suggestions that the toxicity is cumulative, the experts are only going to become busier, not less.
And with a total of 104,510 reports including adults, and an alleged (personal communication confirmed) nine-month wait on autopsies where the death occurred following vaccination, each expert has 4180 reports to review since the beginning of the